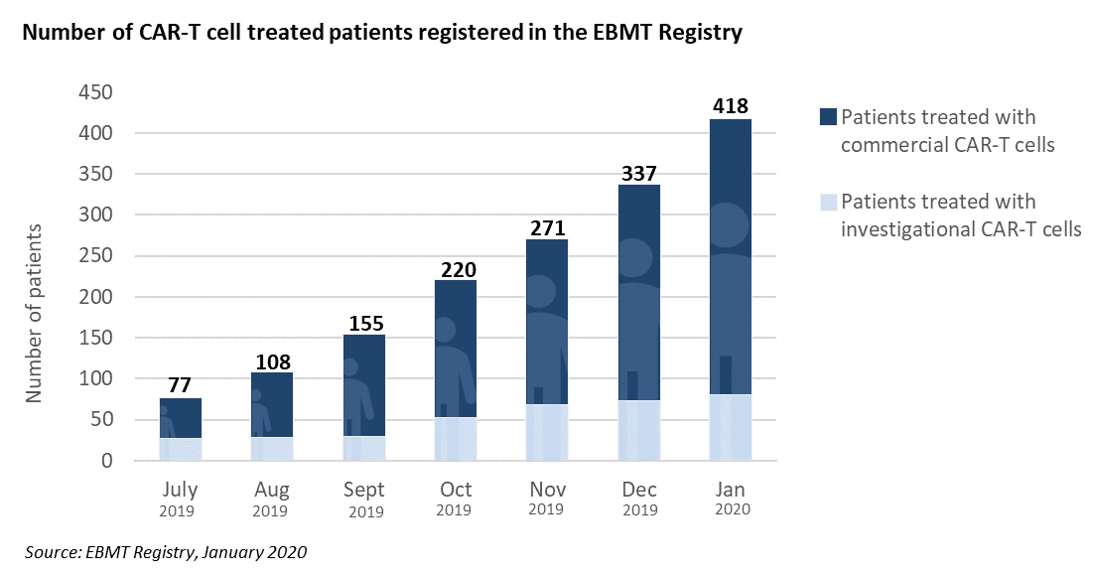
It is now 6 months that EBMT started providing monthly updates on CAR-T cells activities in Europe, less than a year after the first two autologous CAR-T cells gene therapy medicinal products were approved by the European Medicines Agency (EMA), and a time when pricing and reimbursement negotiations were still ongoing in several EU countries.
The information is published in the EBMT Newsletter distributed to its members, and is also freely accessible through the EBMT website at ebmt.org/ebmt/newsletters. It provides the cumulated number of patients treated with CAR-T cells that are registered in the EBMT Registry as currently operated in ProMISe. Numbers are split by commercial or investigational CAR-T cell medicinal products. A map displays countries where registration of clinical activity has started. Figures demonstrate a robust growth in the use of commercial products in most Western European countries, and a significant R&D activity that is ongoing at a growing number of academic centers affiliated to the EBMT. EBMT will continue to provide updated information on a regular basis.
As part of its commitment with many stakeholders interested in the field of immune effector cells based therapies, including EMA, EBMT will pursue its efforts and support the membership to register patients and collect follow-up data; this is currently possible by updating the Cellular Therapy Form that is currently accessible online in ProMISe and further technological advancements of the Registry such as moving in the near future from ProMISe to MACRO. Based on these existing data, EBMT will shortly prepare its first interim report to EU authorities.
Through these efforts, EBMT fulfils its obligations to contribute to a fair and transparent evaluation of this new class of therapeutic products. Our global community needs a thorough assessment of the safety profile and efficacy of these gene therapies in real-world conditions, and thus of their medical value and role in an increasingly complex armamentarium that is available to treat hematological malignancies at large. This can be done only if, patients and patients advocates, health technology assessment agencies, competent authorities, disease specialists as represented by national or transnational cooperative groups, national transplant societies, pharma companies and payers, collaborate. EBMT has started a global initiative to establish such a consortium, with the goal of providing a formal framework for these collaborations, including access to and use of Registry data. To further build on this community, various stakeholder meetings are planned during this year. The next one will take place at the 2nd European CAR T cell Meeting in Sitges, Spain.
Please report all CAR-T cell patients into the EBMT Registry.