
The Transplant Activity Survey has gathered crucial hematopoietic stem cell transplant (HCT) and cellular therapy patient data in Europe for more than 30 years. Since 2024, the EBMT Activity Survey Team in Leiden has taken over managing the Transplant Activity Survey, in anticipation of the forthcoming retirements of Helen Baldomero and Jakob Passweg from Basel, Switzerland. As part of this transition, EBMT introduced a modernised, online version of the Activity Survey.
The new online survey was launched in December 2023 to collect data from 2023 activities to over 700 centres. This development marks a significant milestone for EBMT, as the new platform incorporates user-friendly features, such as automated counts, improved data accuracy, and the ability to save, download or print the form. Moreover, with the new online form, the data collection procedure is faster and more efficient. Currently, the 2023 data collection is closed, and work on the upcoming publication has already started. In the first year following the launch of the online form, 88.5% of the submissions were received through the online platform, marking a significant achievement.
To further improve the EBMT Transplant Activity Survey, the team conducted a satisfaction survey to gather valuable feedback from participating centres. The satisfaction survey sought feedback on the new online platform’s functionality, the survey content, and the support provided by the team. Participants could participate anonymously if they wished to do so.
This satisfaction survey targeted the 678 centres that had already submitted their 2023 activity data by the end of June. It was open for five weeks, yielding 318 responses from 38 countries—a response rate of 47%. 280 (88 %) out of 318 stated that they had used Jotform to fill out the activity survey for their centre.
Participants rated 8 statements on a scale from 1 (Strongly Disagree) to 10 (Strongly Agree), with 5 indicating a Neutral/Undecided stance. There were also open questions where participants add comments or suggestions regarding the online platform, the contents of the survey and the support provided by the team.
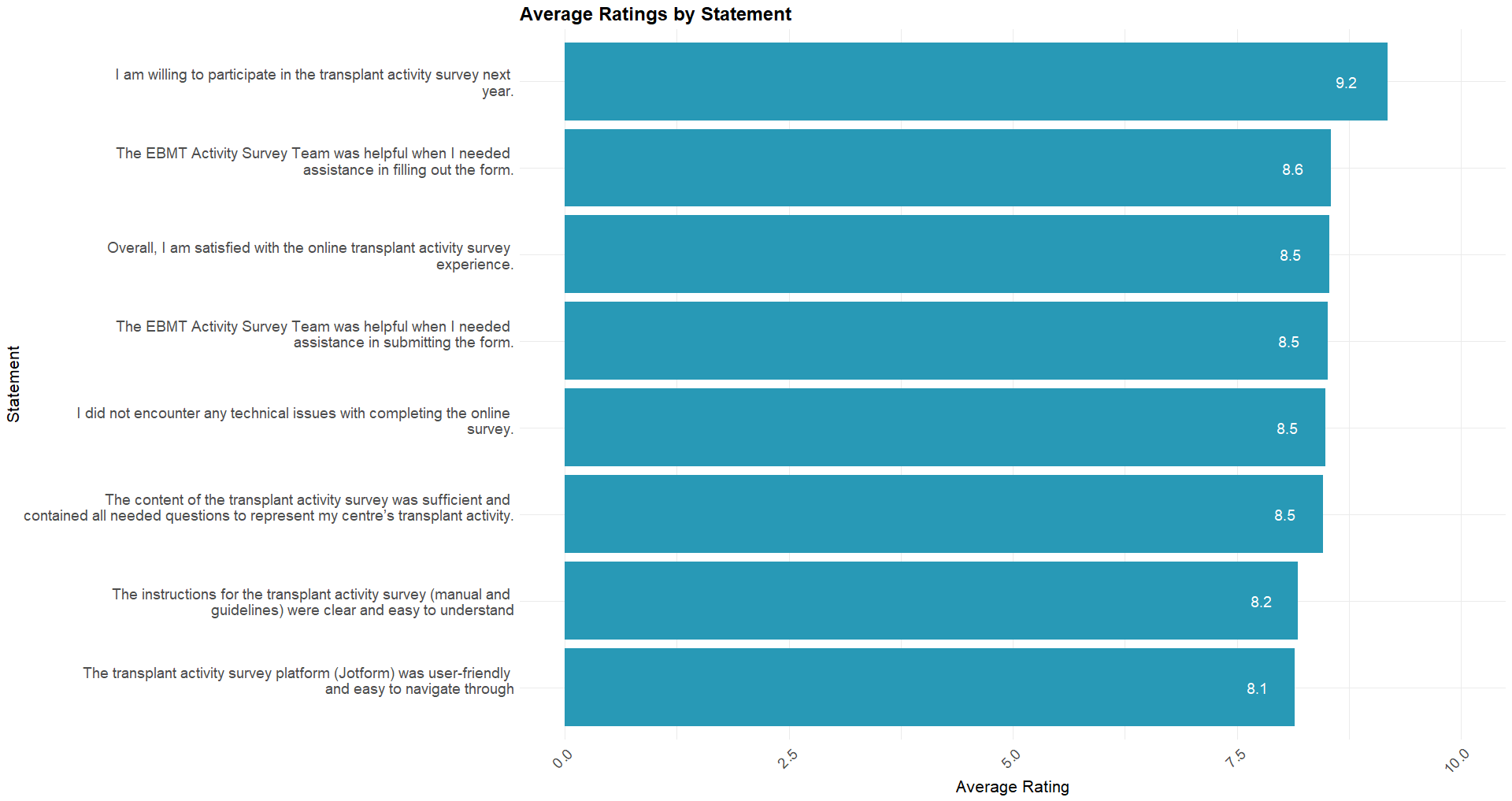
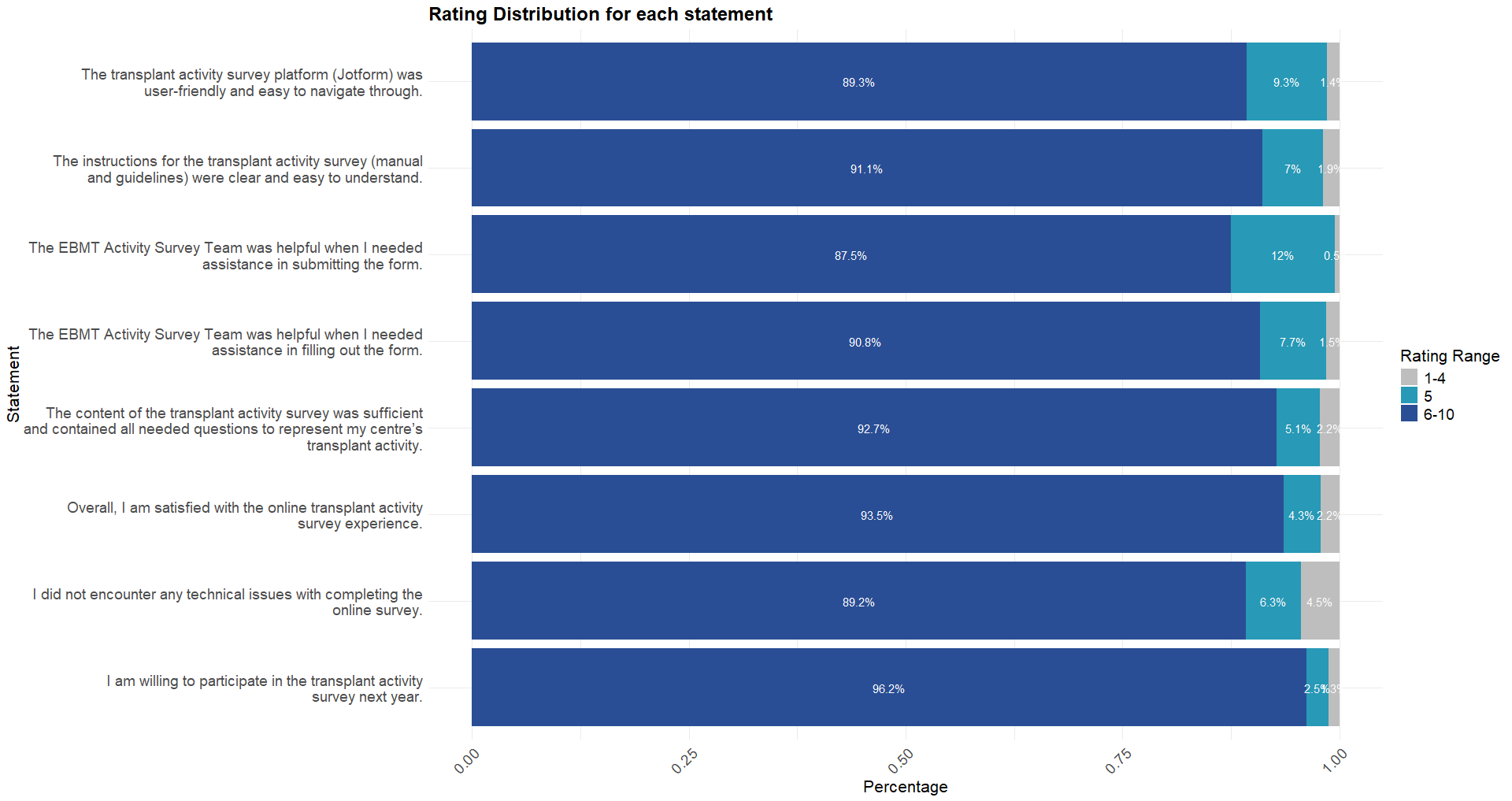
The most common response (mode) across all statements was 10. 92% of the responses were positive (rating of 6-10), 6% were neutral (rating of 5), and 2% (rating 1-4) were negative. The average ratings range between 8.1 and 9.2, indicating that, on average, participants strongly agreed with all 8 statements. The statement with the highest average rating was, “I am willing to participate in the transplant activity survey next year.” with an average rating of 9.2. In contrast the statement with the lowest average rating (8.1) was, “The transplant activity survey platform (Jotform) was user-friendly and easy to navigate through”.
The comments and suggestions received from the open questions revealed that the majority of them (33%) were related to the print out of the online form. Many users found the ability to export a PDF of their completed survey valuable, but also noted areas for improvement in the printout functionality. 20% of the comments provided suggestions for improvements to the online form's interface, as well as feedback on the medical content of the survey. Participants suggested that some patient diagnoses are not completely clear or that specific diagnoses are missing from the form. 7% of the comments were related to technical problems the participants faced while accessing or filling out the online form. 9% of the comments were related to the assistance provided by the team.
Overall, participants appreciated the new platform's ease of use and the responsive support from the team, as highlighted by comments like: "Easy to use, much more convenient than the old way. Congratulations” and “We had one question, which we asked via e-mail, and received quick and kind reply”.
Based on the feedback received through the satisfaction survey, the EBMT Activity Survey Team has developed an action plan to enhance the survey experience. Our top priority is to improve the printout feature, ensuring that users receive a well-formatted and easy-to-read document for the 2024 survey. Additionally, we have carefully considered the medical suggestions provided and will incorporate them into next year’s survey if needed. Lastly, the team will work hard to provide better documentation (manual and guidelines) for the upcoming survey.
We are excited about the future of the survey and are committed to continuous improvement. We greatly appreciate the participation of all centres in the satisfaction survey and their valuable feedback, which play a crucial role in shaping our efforts moving forward. Your ongoing support is highly valued, and we look forward to continuing our collaboration for the 2024 survey.
For questions, contact us via email at activitysurvey@ebmt.org