
June 2024 Clinical Case of the Month
Title: Refractory Autoimmune Haemolytic Anaemia (AIHA) after Allogeneic Stem Cell Transplantation: stabilisation with the use of rituximab and bortezomib.
Submitted by: Dr Jose Maria Aspa-Cilleruelo
Physicians' expert perspective: Dr Elisa Roldan-Galvan & Professor John Snowden
Sheffield BMT and Cellular Therapy Programme, Department of Haematology, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
A 67-year-old male patient diagnosed with severe aplastic anaemia was initially treated with horse anti-thymocyte globulin (h-ATG, ATGAM) and ciclosporin (CsA) without response. He subsequently underwent allogeneic haematopoietic stem cell transplant (HSCT) from a 10/10 HLA-matched and ABO and CMV-matched unrelated male donor (MUD) with reduced intensity conditioning consisting of fludarabine, cyclophosphamide and alemtuzumab in September 2021. At day +100, he showed complete recovery of the full blood count and full donor chimerism, with no graft-versus-host disease (GVHD) and subsequently he maintained stable mixed chimerism and blood counts sufficient to commence weaning of CsA starting at around 11 months post-transplant.
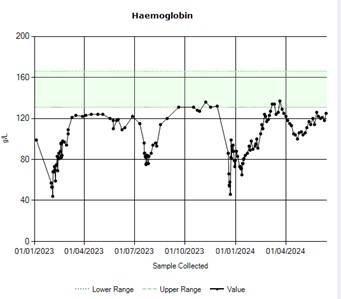
Fifteen months post-transplant, still under CsA treatment due to mixed chimerism, the patient presented with significant fatigue and an isolated drop in haemoglobin level (Figure 1), with blood film and other laboratory parameters consistent with autoimmune haemolysis and immuno-haematolgical workup confirming a positive direct Coombs test (IgG and C3), but no evidence of red cell antigen specificity on allo- or auto-antibody testing. Chimerism was 81%. No PNH clone was detected and there were no signs of relapse in the aspirate and the trephine biopsy. Infectious screening, including CMV, EBV and parvovirus, was negative. A CT thorax-abdomen-pelvis excluded other underlying pathology. A diagnosis of autoimmune haemolytic anaemia (AIHA) post-allogeneic transplant was made, and the patient was started on steroids (prednisolone 1 mg/kg) and intravenous immunoglobulins (1g/kg/day for 2 days). After 7 days without response, rituximab 375 mg/m² weekly was added, resulting in improvement in both haemoglobin level and haemolytic markers after 4 doses.
Six months later, during the reduction of the prednisolone dose (<20 mg/day), the patient experienced a relapse requiring hospitalisation. Mycophenolate mofetil (MMF) was added as a steroid-sparing agent, and ciclosporin was switched to sirolimus. Due to gastrointestinal intolerance, MMF was discontinued, followed by another mild AIHA episode requiring 4 additional doses of rituximab.
In November 2023, nearly two years after the diagnosis, the patient required hospitalisation for another episode of AIHA again during the steroid reduction, confirming his steroid dependence. At this stage, splenectomy was discussed as the next standard option (as per non-HSCT settings). The patient received encapsulated vaccinations and he was reviewed by the general surgeons. However, patient suffered pulmonary emboli and requiring high dose anticoagulants, effectively precluding surgery at that time.
At this stage, the case was discussed in a UK national expert immune-haematology MDT meeting who recommended re-starting rituximab in combination with bortezomib (1.3 mg/m²) weekly. With stable sirolimus levels and good tolerance to bortezomib, the patient eventually achieved stable haemoglobin levels and improved haemolytic parameters. He completed 8 cycles of bortezomib with no related toxicity and good response. He continues a slowly tapering dose of prednisolone. Moving forward, the plan is to reduce the immunosuppression with sirolimus and continue with rituximab every 6 months.
Which of the following is not a therapeutic option in refractory autoimmune haemolytic anaemia?
A. Bortezomib
B. Daratumumab
C. Ibrutinib
D. Ruxolitinib
E. Splenectomy
Expert perspective by Elisa Roldan-Galvan and John Snowden:
AIHA is an occasional but well-recognised complication after allogeneic transplantation and, given the clinical complexity of the post-transplant setting, is frequently challenging in its presentation and management. In addition to ruling out other causes of haemolytic anaemia, such as infections, TMA, drugs and PNH, extensive work up is necessary including assessment of primary transplant indication, GVHD or other complications related or unrelated to the transplant process.
Identified risk factors for developing this complication include the use of alemtuzumab (Campath) and fludarabine use in the conditioning regimen, CMV reactivation after transplant, the use of cord blood as a progenitor source, and a history of non-malignant disease as indication for transplant. Secondary autoimmune diseases are increasingly recognised in primary transplant indications with an autoimmune component (such as aplastic anaemia, as in this case), but are also an occasional complication of malignant and other indications.
The treatment of post-transplant AIHA is based on retrospective studies in limited populations and expert recommendations, with no randomized clinical trials available, leading to different approaches depending on centre experience and access to immunomodulatory drugs and other treatments.
Initial therapy in most postr-transplant cases mirrors that of non-transplant-related AIHA. From the start of immunosuppressive treatment, a high level of vigilance and potentially additional measures are mandatory to prevent/minimise infection in these already immunocompromised and variably deconditioned patients. In contrast to the non-transplant setting, the use of steroids alone or in combination with intravenous immunoglobulins has shown relatively low effectiveness, less than 50%, in these patients, and it is common to move onto adjunctive immunosuppressants. Classical immunosuppressive treatments such as MMF, azathioprine, mercaptopurine, cyclophosphamide, or more invasive methods like splenectomy or plasma exchange have limited published experience in the context of allogeneic HSCT and are generally based on the physician's experience and individualised patient preferences.
A better understanding the pathophysiology of AIHA has led to small series trials with biological treatments in cases of loss of response or refractoriness to Rituximab, aiming to attenuate the immune response at different points of cellular immunity. Published experiences include using both bortezomib and daratumumab, with the former showing promising results in a phase 2 prospective study presented at ASH 2023 confirming high rate of responses and good safety profile. Combination of these new agents with standard immunosuppression is probably the best option to get a good response in complex, refractory, steroid dependent patients.
This case illustrates the challenges of this complication, difficulties with primary management leading to combining different immunosuppressive agents (Rituximab, Bortezomib, and Sirolimus) to achieved current stabilisation in this patient, who, despite his age and comorbidities, attendant complications (including thrombo-embolism associated with chronic haemolysis) and overall duration of illness, has maintained an acceptable quality-of-life to date.
This case also highlights the value of ongoing review of a national immune-haematology panel, where pooled experience from a broader range of experts (non-transplant and transplant) was valuable in establishing longer term stabilisation, which we hope will be uncomplicated as immunosuppression is tapered. However, there remains a need for evidence-based consensus and harmonisation of clinical and laboratory practice in these occasionally challenging patients, and for future research assessing the value of modern approaches to AIHA in the post-transplant setting.
Correct Answer: D or E
References:
- Killick, S. B., Bown, N., Cavenagh, J., Dokal, I., Foukaneli, T., Hill, A., Hillmen, P., Ireland, R., Kulasekararaj, A., Mufti, G., Snowden, J. A., Samarasinghe, S., Wood, A., & Marsh, J. C. W. (2016). Guidelines for the diagnosis and management of adult aplastic anaemia. *British Journal of Haematology, 172*(2), 187-207. doi: https://doi.org/10.1111/bjh.13853
- Soe ZN, Karakantza M, James B, Clay J, Adams A, Gilleece MH. Autoimmune Haemolytic Anaemia after Allogeneic Haematopoietic Stem Cell Transplantation; Single Centre Experience. Blood. 2019;134(Supplement_1):5675. doi: https://doi.org/10.1182/blood-2019-131951
- Miller, P. D. E., Snowden, J. A., De Latour, R. P., Iacobelli, S., Eikema, D. J., Knol, C., Marsh, J. C. W., Rice, C., Koh, M., Fagioli, F., Chaganti, S., Finke, J., Duarte, R. F., Bader, P., Farge, D., Passweg, J. R., Madrigal, J. A., & Dufour, C. (2020). Autoimmune cytopenias (AIC) following allogeneic haematopoietic stem cell transplant for acquired aplastic anaemia: a joint study of the Autoimmune Diseases and Severe Aplastic Anaemia Working Parties (ADWP/SAAWP) of the European Society for Blood and Marrow Transplantation (EBMT). *Bone Marrow Transplantation, 55*(2), 441-451. doi: 10.1038/s41409-019-0680-4.
- González-Vicent M, Sanz J, Fuster JL, Cid J, Díaz de Heredia C, Morillo D, Fernández JM, Pascual A, Badell I, Serrano D, Fox L, de la Serna J, Benito A, Couselo JM, Molina B, Díaz MA, Sanz MA. Autoimmune hemolytic anemia (AIHA) following allogeneic hematopoietic stem cell transplantation (HSCT): A retrospective analysis and a proposal of treatment on behalf of the Grupo Español De Trasplante de Médula Ósea en Niños (GETMON) and the Grupo Español de Trasplante Hematopoyetico (GETH). Transfusion Medicine Reviews. 2018;32(3):179-185. doi: https://doi.org/10.1016/j.tmrv.2018.02.005
- Barcellini W, Fattizzo B. How I treat warm autoimmune hemolytic anemia. Blood. 2021;137(10):1283-1294. doi: https://doi.org/10.1182/blood.2019003808
- McGlothlin J, Abeykoon J, Al-Hattab E, Ashrani AA, Elliott M, Hook CC, Pardanani A, Pruthi R, Sridharan M, Wolanskyj A, Rouse R, Go R. Bortezomib and daratumumab in refractory autoimmune hemolytic anemia. Am J Hematol. 2023;98:E263-E265. doi: https://doi.org/10.1002/ajh.27025
- Lin X, Yang C, Zhuang J, Zhou D, Chen M, Han B. Rituximab Plus Bortezomib for Refractory and Relapsed Warm Autoimmune Hemolytic Anemia: a Prospective, Single-Arm, Phase Ⅱ Trial. Program: Oral and Poster Abstracts, Session: 101. Red Cells and Erythropoiesis, Excluding Iron: Poster III, 2023 ASH Annual Meeting; 2023 Dec 11.
- Jalink, M., Jacobs, C. F., Khwaja, J., Evers, D., Bruggeman, C., Fattizzo, B., Michel, M., Crickx, E., Hill, Q. A., Jaeger, U., Kater, A. P., Mäkelburg, A. B. U., Breedijk, A., te Boekhorst, P. A. W., Hoeks, M. P. A., de Haas, M., D’Sa, S., & Vos, J. M. I. (2024). Daratumumab monotherapy in refractory warm autoimmune haemolytic anaemia and cold agglutinin disease. *Blood Advances, 8*(11), 2622–2634. doi: https://doi.org/10.1182/bloodadvances.2024012585
Acknowledgements:
We recognise the support of Professor Nicola Cooper and the UK National Immuno-Haematology MDT. We acknowledge the patient, who has given permission for his case to be discussed, with anonymised identifiers.
Future Clinical Case of the Month
If you have a suggestion for future clinical case to feature, please contact Anna Sureda.