JACIE Activity Report 2024


JACIE continued to work towards its goal – to harmonise and continually improve quality control standards in cellular therapy in Europe and beyond. In this, JACIE has continued to make strides in increasing its reach and activity with a record number of inspections carried out in 2024. This is thanks to the nearly 300 inspectors and the committee members, who have given their time generously to the benefit of the Cellular Therapy community.
There is still a lot to do. JACIE will continue the development of the 9th Edition of HCT and the 3rd Edition of IEC Standards in collaboration with the colleagues from FACT. We also know that the advancements in cellular therapies and new technologies promise a dynamic future for JACIE as we strive to build a stronger, more inclusive community.
Activity update
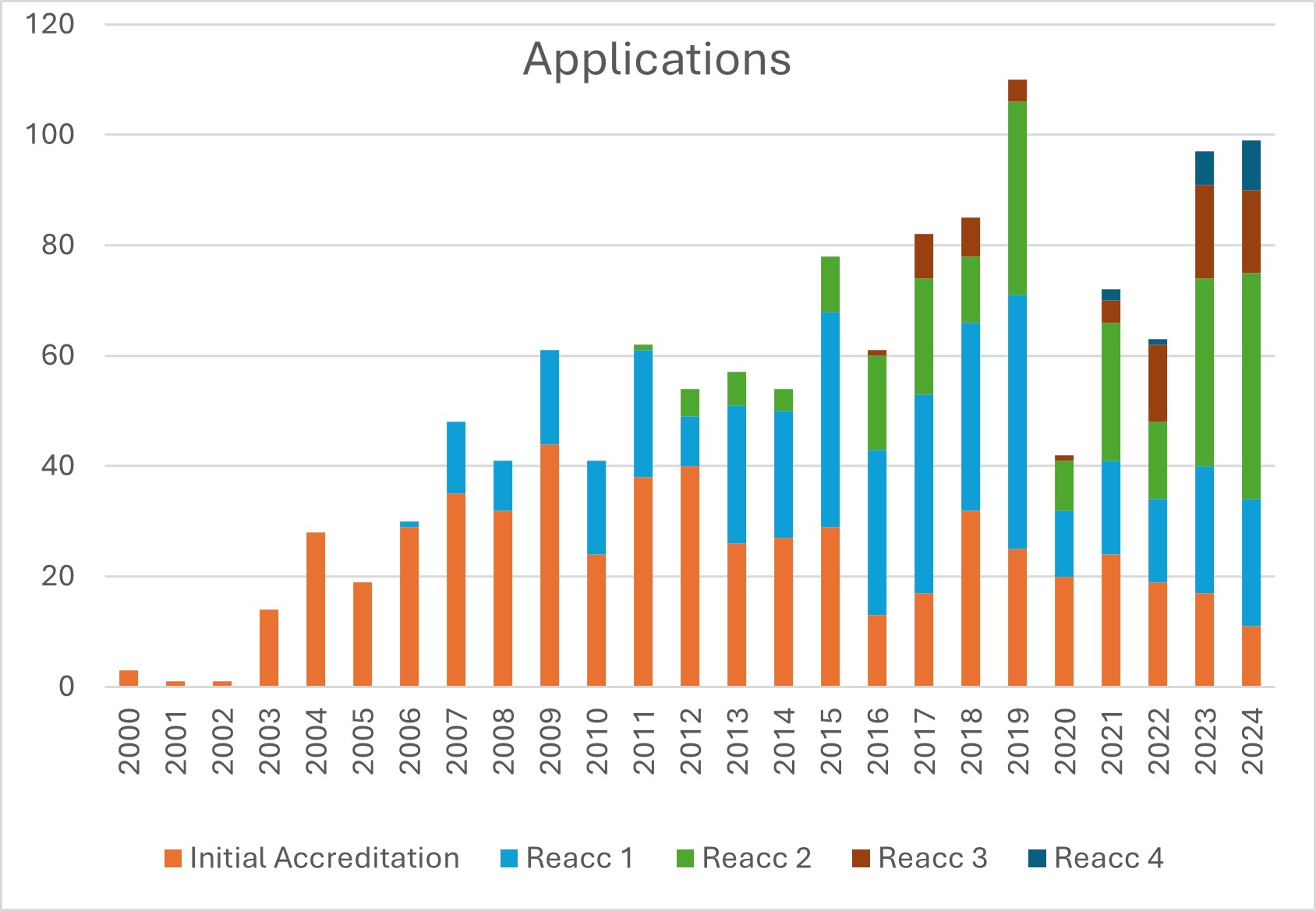
JACIE’s activity continued a strong post-covid performance. There were 99 applications in 2024, with 11% of the centres applying for initial accreditation and 9% applying for their 4th re-accreditation. Although JACIE remains firmly embedded in the European Cellular Therapy community, there is an increasing interest in JACIE in Middle East and further afield accounting currently approximately 5% of the applications.
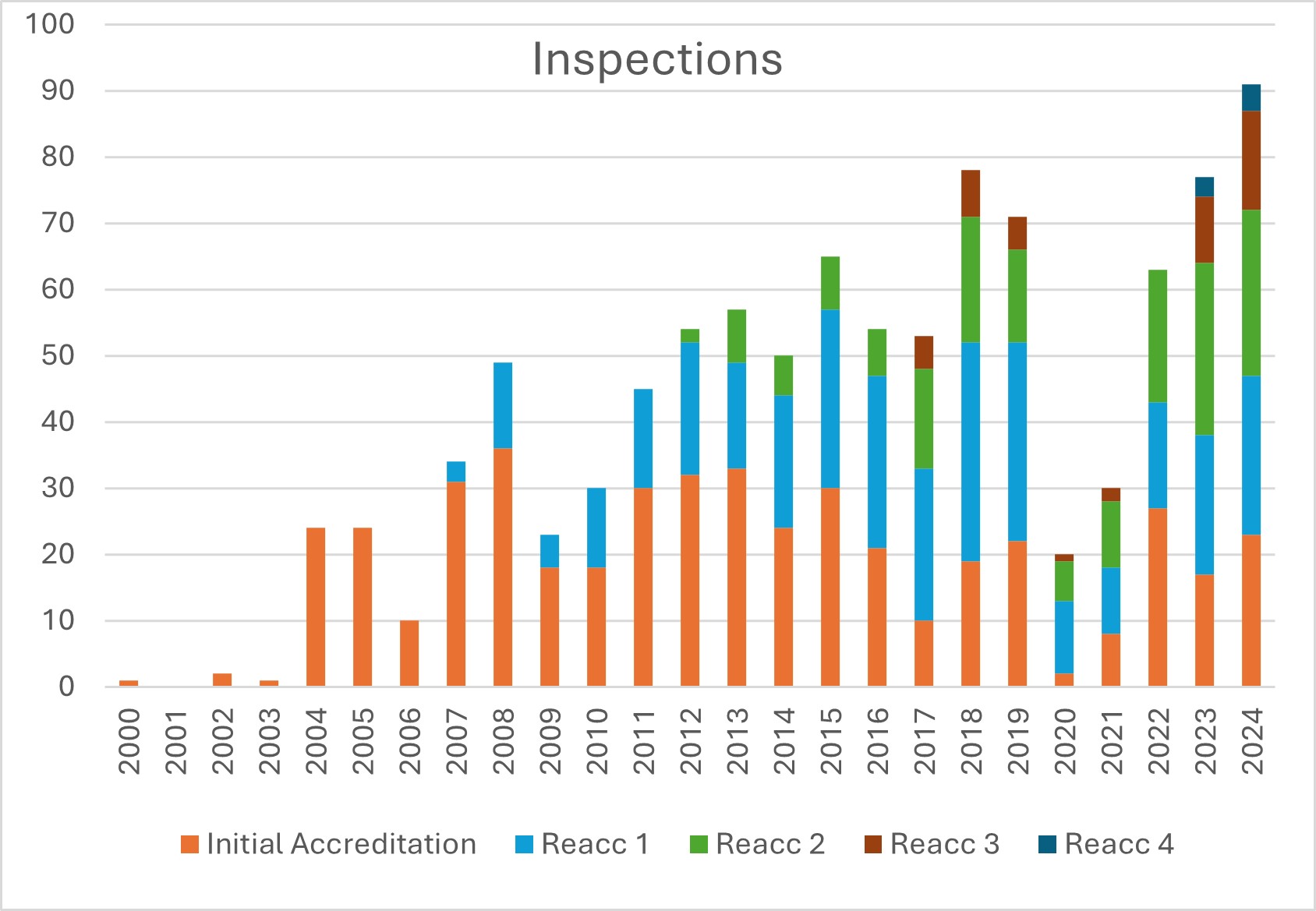
JACIE carried out 91 inspections in 2024, which is the highest number of inspections in the history of JACIE (2023: 77 inspections). This is only possible thanks to the commitment of our inspectors; many of whom have carried out two or even three inspections in the course of the year.
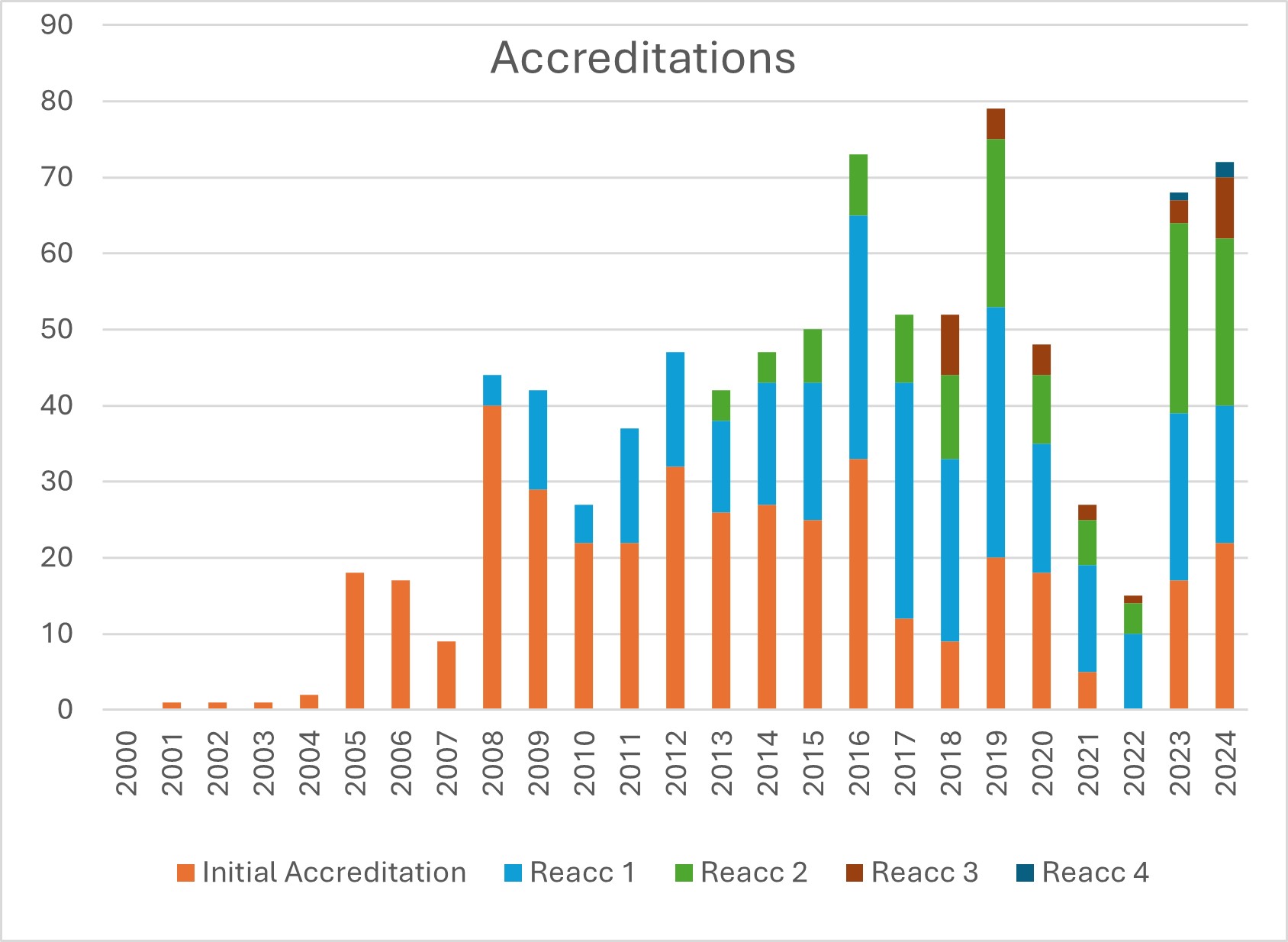
There was a slight increase in the number of centres accredited in 2024 with 72 Centres completing the process in 2024. This reflects the collaborative efforts of the Accreditation Committee, which continued to meet fortnightly to review all the inspection reports, and the applicants responding in a timely manner to deficiencies identified during the inspections.
Institutions accredited in 2024
| Facility | Institution | City | Country |
|---|---|---|---|
| Centro Trapianti di Cellule Staminali Emopoietiche Reggio Emilia | AUSL di Reggio Emilia-IRCCS | Reggio Emilia | Italy |
| Cellular and Molecular Therapies (CMT), NHS Blood and Transplant, Southampton | NHS Blood and Transplant | Southampton | United Kingdom |
| Programme de greffe de CSH_Hématologie clinique - Hémobiothérapie | Hôpital Pitié-Salpêtrière APHP Sorbonne Université DMU ORPHé | Paris | France |
| The autologous hematopoietic stem cell transplantation program for Department of Hematology and Department of Clinical Immunology | Odense University Hospital | Odense | Denmark |
| Programma Trapianto ASST Valle Olona | ASST Valle Olona Bursto Arsizio | Busto Arsizio | Italy |
| Seracell Pharma GmbH Cell processing-minimally manipulated | Seracell Pharma GmbH | Rostock | Germany |
| Manchester Royal Infirmary Adult Haematology Stem Cell, Cellular & Gene Therapies Unit | Manchester University NHS Foundation Trust | Manchester | United Kingdom |
| St Vincent’s Hospital BMT Program | The Kinghorn Cancer Centre, St Vincent’s Hospital | Sydney | Australia |
| University Hospitals Bristol and Weston NHS Foundation Trust Stem Cell Transplant and Cellular Therapy Programme | University Hospitals Bristol NHS Foundation Trust | Bristol | United Kingdom |
| King’s College Hospital NHS Foundation Trust - Department of Haematological Medicine | NHS Foundation Trust | London | United Kingdom |
| South West Peninsula Transplant Service | University Hospitals Plymouth NHS Trust, Plymouth, and Royal Cornwall Hospitals NHS Trust, Truro | Plymouth | United Kingdom |
| HSTP- GZA | GZA hospitals campus Sint Augustinus | Wilrijk | Belgium |
| Programma Trapianto | Istituto Nazionale Tumori Fondazione G.Pascale | Napoli | Italy |
| Gloucestershire Haematopoietic Stem Cell Transplant Service | Gloucestershire Hospitals NHS Foundation Trust | Cheltenham | United Kingdom |
| Zentrum für Stammzell- und immunologische Zelltherapie (ZSIZ) | Klinik für Hämatologie, Onkologie, Stammzelltransplantation und Zelltherapie Universitätsklinikum Knappschaftskrankenhaus Bochum | Bochum | Germany |
| Programma Trapianto CSE | Azienda Ospedaliero-Universitaria di Modena | Modena | Italy |
| UHL HPC Transplant Programme Leicester Royal Infirmary, University Hospital of Leicester NHS Trust (UHL) and Northampton General Hospital NHS Trust (NGH) | Leicester Royal Infirmary | Leicester | United Kingdom |
| Klinik I für Innere Medizin and Pädiatrische Onkologie und Hämatologie | Universitätsklinikum Köln (AöR) | Cologne | Germany |
| MEDYAG Kft. | MEDYAG Kft. | Debrecen | Hungary |
| BMT and CART programme | Great Ormond Street Hospital for Children NHS Foundation Trust | London | United Kingdom |
| Kliniken Essen-Mitte || Klinik für Hämatologie | Int. Onkologie | Stammzelltransplantation | Evangelisches Krankenhaus Essen-Werden | Essen | Germany |
| Paediatric Hematopoietic Stem Cell Transplantation Unit & Immune Effector Cell (CAR-T) paediatric Unit | Hospital Sant Joan de Déu | Esplugues de Llobregat | Spain |
| Center of Oncology/Hematology and Transfusion Medicine, Kantonsspital Aarau, Switzerland | Kantonsspital Aarau AG | Aarau | Switzerland |
| Programme de transplantation de cellules souches hématopoïétiques adulte et pédiatrique | Cliniques Universitaires Saint-Luc (CUSL) | Brussels | Belgium |
| BST - Hospital Dr. Josep Trueta Girona | Banc de Sang i Teixits | Girona | Spain |
| BST - Hospitalet de Llobregat-Bellvitge | Banc de Sang i Teixits | Hospitalet de Llobregat | Spain |
| BST - Hospital Sant Joan de Déu | Banc de Sang i Teixits | Esplugues de Llobregat | Spain |
| BST - Hospital Germans Trias i Pujol | Banc de Sang i Teixits | Badalona | Spain |
| BST - Hospital Sant Pau | Banc de Sang i Teixits | Barcelona | Spain |
| Stem Cell Transplantation/ IEC Programme University Medical Centre Utrecht and Sanquin (including Wilhelmina Children’s Hospital WKZ - Princess Máxima Center for pediatric oncology joint programme) | University Medical Center Utrecht Sanquin Princess Máxima Center | Utrecht | Netherlands |
| Service des Maladies du Sang | CHU Angers & EFS CPDL | Angers | France |
| Wessex Blood and Marrow Transplant & Cellular Therapy | University Hospital Southampton NHS Foundation Trust incorporating: Portsmouth University Hospitals NHS Trust Salisbury District Hospital NHS Foundation Trust | Southampton | United Kingdom |
| CMT Cellex Manufacturing Transports and Logistics GmbH | CMT Cellex Manufacturing Transports and Logistics GmbH | Cologne | Germany |
| Programma Trapianti Cellule Staminali periferiche e midollari AST-PU (Azienda Sanitaria Territoriale Pesaro Urbino) | Azienda Sanitaria Territoriale Pesaro Urbino | Pesaro | Italy |
| Centrum voor Oncologie, Autoloogstamceltransplantatie programma | AZ Turnhout vzw, Campus Sint-Elisabeth | Turnhout | Belgium |
| University Medical Centre Wuerzburg, Institute for Clinical Transfusion Medicine and Hemotherapy (IKTH), Apheresis Collection and Cell Processing Program | Institute for Clinical Transfusion Medicine an Hemotherapy (IKTH), Universitätsklinik Würzburg | Würzburg | Germany |
| Centro Trapianto Adulti | Ospedale San Gerardo | Monza | Italy |
| NHS Blood and Transplant- Therapeutic Apheresis Services Sheffield | NHS Blood and Transplant, Therapeutic Apheresis Services | Sheffield | United Kingdom |
| Transplant programme at 4th Department of Internal Medicine – Hematology, University Hospital Hradec Králové | University Hospital Hradec Králové | Hradec Králové | Czech Republic |
| Unité d’Hématologie Oncologie Pédiatrique - Hôpital des enfants | CHU de Bordeaux | Bordeaux | France |
| Programa de TPH | Hospital Universitario de Navarra | Pamplona | Spain |
| CHU de Grenoble Alpes | CHU de Grenoble Alpes | La Tronche | France |
| Paediatric BMT Center | Ege University Medical School | İzmir | Turkey |
| Aarhus Center for Hematopoietic Stem Cell Transplantation | Aarhus University Hospital | Aarhus | Denmark |
| Hämatologie, Onkologie und Palliativmedizin und Bereich Blutprodukte – Stammzellherstellung | Robert Bosch Krankenhaus | Stuttgart | Germany |
| Programa de Trasplante de Progenitores Hematopoyéticos del Hospital Clínic de Barcelona | Hospital clinic de Barcelona | Barcelona | Spain |
| Programme de Greffe de Moelle du CHU de Nantes | CHU de Nantes | Nantes | France |
| Autologous Stem Cell Service | Great Western Hospitals NHS Foundation Trust | Swindon | United Kingdom |
| Unidad de Trasplante Hematopoyético | Complejo Hospitalario Universitario de A Coruña | A Coruña | Spain |
| Hospital Universitario Central de Asturias | Oviedo | Spain | |
| Programma di Trapianto Autologo di Midollo Osseo (P-TMO autologo) | Istituto Oncologico Veneto-IRCSS | Padova | Italy |
| Medizinische Klinik mit Schwerpunkt Hämatologie, Onkologie und Tumorimmunologie | Charité Universitätsmedizin Berlin | Berlin | Germany |
| Hematopoietic stem cell transplantation program | Antwerp University Hospital (UZA) | Edegem | Belgium |
| Programa de Trasplante y Terapia Celular | Complexo Hospitalario Universitario de Vigo | Vigo | Spain |
| The Norwegian Stem Cell Transplantation Program. | Haukeland University Hospital | Bergen | Norway |
| The South African National Blood Services Haematopoietic Cellular Therapy Collections and Processing Facilities | The South African National Blood Services | Johannesburg | South Africa |
| Hospital Clínico Universitario de Valencia | Valencia | Spain | |
| The Stem Cell Transplantation and Cellular Therapy Unit | The Clatterbridge Cancer Centre NHS Foundation Trust | Liverpool | United Kingdom |
| Bone Marrow Transplantation Unit | University Hospital of Patras | Rio Patras | Greece |
| Haematopoietic Stem Cell Transplant Unit, Aberdeen Royal Infirmary | NHS Grampian | Aberdeen | Scotland |
| Programma Trapianti Dipartimento Oncologico | La Maddalena S.p.A. | Palermo | Italy |
| UOC Ematologia | ASST Papa Giovanni XXIII – Hematology and Bone Marrow Transplant Unit | Bergamo | Italy |
| Programa de Trasplante y Terapia Celular | Institut Català d´Oncologia, Hospital Duran i Reynals | L'Hospitalet de Llobregat | Spain |
| Marien Kliniken Department of Hematology, Oncology and Stem Cell Transplantation | Marien Gesellschaft Siegen gGmbH | Siegen | Germany |
| AO San Camillo Forlanini, UOC EMATOLOGIA E TRAPIANTI CELLULE STAMINALI | AO San Camillo Forlanini | Rome | Italy |
| Department of Haematooncology | University Hospital Ostrava | Ostrava | Czech Republic |
| Stem Cell Transplantation Unit, Department of Hematology | Helsinki University Hospital Comprehensive Cancer Center | Helsinki | Finland |
| Centro Unico Trapianti di CSE Adulti e Pediatrico | ARNAS G.Brotzu | Cagliari | Italy |
| Fondazione IRCCS Policlinico San Matteo | Pavia | Italy | |
| Department of Medicine II, Sektion Stammzell-Transplantation | Universitäts-Klinikum Augsburg | Augsburg | Germany |
| Department of Transplantation | University Children's Hospital of Krakow | Krakow | Poland |
| Programme lausannois de transplantation de CSH autologues | CHUV | Centre hospitalier universitaire vaudois | Lausanne | Switzerland |
JACIE Inspectors
In 2024, three Inspector Training Courses took place in Barcelona, Cambridge and Stockholm with a total of 59 new inspectors trained. The training days are always highlights in the JACIE calendar and we would like to thank BSBMTCT JACIE Committee and the Nordic Transplant Group for their support for these training events.
JACIE continued the grants programme to the inspectors to attend the 50th Annual Meeting of the EBMT in Glasgow, enabling valuable opportunities for learning and collaboration. A total of 72 grants were awarded to attend the meeting either in-person or virtually. Additionally, as part of JACIE’s commitment to enhance inspector incentives, the Per Diem for inspections was increased from €80/day to €120/day to ensure that the inspectors will not incur any expenses during the inspection visits. Lastly, the Inspector Committee (JIC) continued to lead Inspectors' Webinars providing regular updates both on the Standards and accreditation process to the inspectors.
JIC is an important stakeholder committee representing the Inspectors, a key stakeholder group in the accreditation process. Following an open call for Expressions of Interest, the Inspector Committee welcomed new members as well as a new Chair as Ms Eugenia Trigoso stepped down as the Chair after two busy and successful years in the lead.
9th Edition Standards review process
JACIE continued in 2024 the collaboration with FACT (Foundation for Accreditation in Cellular Therapy) to develop the 9th edition of the HSC Standards and the 3rd edition of the Immune Effector Cells (IEC) Standards.
FACT-JACIE Steering Committee met in San Antonio, Texas, USA in February to review the drafts developed in the Sub-Committees for Clinical, Collection, Processing and Quality Management Standards. After further review by the sub-committees, the Standards were open for Public consultation the 3rd quarter of 2024. The Sub-Committees will review the comments received and the prepare the final version for publication in the course of 2025.
JACIE Centres
JACIE is committed to supporting Centres interested in any of the stages of the accreditation process. This work is led by the Quality Managers Committee (QMC). The Committee welcomed new members in 2024 and also said a fond farewell to Mrs Renza Monteleone whose leadership has been vital in developing the Quality Management days in the EBMT Annual Meetings.
Following the successful introduction of the E-learning programme for Centres end of 2023, JACIE introduced quarterly Drop-in Sessions for Centres. These sessions are designed for both Centres beginning their JACIE journey and those already in the process. They provide a valuable opportunity to connect directly with the JACIE team, gain insights, and have questions about the Accreditation process answered.
Finally, JACIE made a transition from paper certificates to digital certificates in 2024. These certificates are now provided in a safe, digital format and can also be verified online for added convenience and security. For Centres that prefer a physical copy, the digital certificates can be easily downloaded as high-resolution PDFs for printing. This shift not only streamlines processes but also aligns with our commitment to reducing environmental impact.



