The EBMT Registry

In 2024, the EBMT Registry has seen various improvements since its release in August 2023. Monitoring functionalities were added (including source document verification tracking), versioning of forms was enabled, fields for extended dataset data were created, the study manager tool was launched (to be piloted) and most importantly, MicroStrategy was released for downloading data. In total, there were nine releases over the year. Unfortunately, due to the need for these developments, it has not been possible for the EBMT Working Parties to perform studies using new Registry data.
Migrations of remaining core dataset items continued throughout 2024, and the registry team hopes to finish all migrations, including selected extended data, by the end of 2025. The core dataset forms, which were released in 2023, have undergone a review and are now in a the 2nd version. Additionally, the extended dataset forms were released for data entry in November.
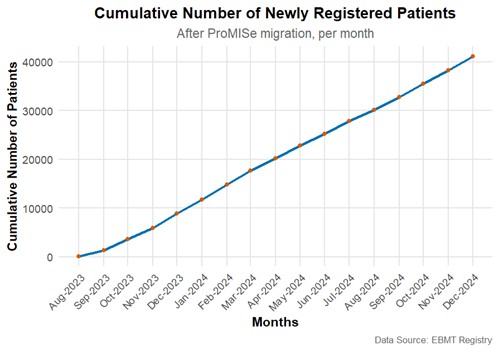
The EBMT Registry has been accessed by ±550 unique users during 2024. These users, in total, created 32,949 new patients. Since the EBMT Registry went live in August 2023, 43,859 HCTs were entered, of which 45% were allogeneic. 6,424 cellular therapies and 34 gene therapies were entered as well.
The Registry team has also spent time improving our educational materials by creating a MicroStrategy e-learning course and a HLA data entry manual. There were also various Q&A sessions where users could ask their medical and form-related questions to our physicians.
The Registry Committee has held monthly meetings to discuss ongoing business and troubles reported from various stakeholders. In addition, every two months, EBMT held meetings with the national registries to update them on the status of the Registry and answer all sorts of questions. Through these channels, the registry team collected practical feedback from many users. In addition, the Registry Newsletter with updates, including the status of our EuroTractor Grant, was distributed to all our members on a monthly basis.
A lot of time and effort has been devoted by the EBMT team, National Registries, and the Registry Committee to assess areas of improvement and act on those areas. The EBMT Registry has developed from a novel data entry platform to a more stable environment with more sophisticated functionalities. The main priority for 2025 will be to start up several EBMT studies using the data from the new Registry. During 2025 we will also continue our work with various developments and improvements, including data exporting and the release of the study tool for additional study data collection.

